Tissues / Ocular Tissues & Fluids
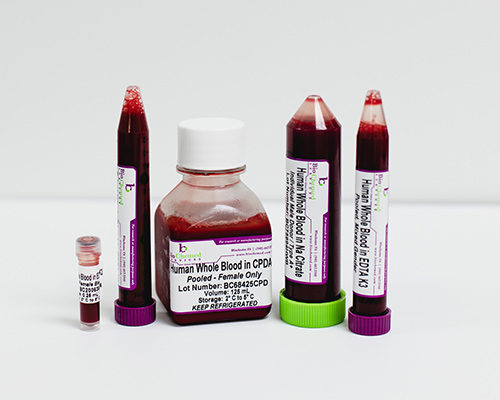


Normal human blood products are collected from consented donors under IRB approved protocols at facilities located in the continental United States. We use current Standard Operating Procedures and offer all commercially available anticoagulants. This allows us to customize every order according to your exact specifications.
Inclusion and/or exclusion criteria can be specified such as age, gender, weight, ethnicity, smoking status, etc. Specific questions (i.e. use of certain medications) can also be added to the product requirements to enable customized screening of donors.
Biochemed Services provides viral negative normal human blood-derived samples and will be noted on the Certificate of Analysis included with each shipment.
We offer top-quality, fully-customized biological products and services. Give us your specifications, and we will provide you with a detailed quote. View our complete product catalog